توضیحات
Chemical Formula of Ferrous sulfate: Fe2(SO4)3
Ferrous Sulfate Crystal with a purity of 18%
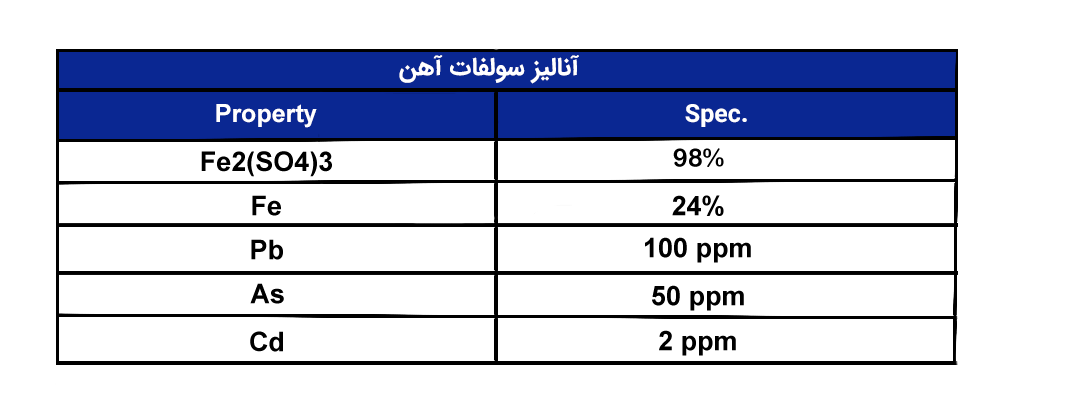
Powdered Ferrous Sulfate with a purity of 24%
Ferrous sulfate Trihydrate with a purity of 28%
Other Names: Green Vitriol
Packaging of Ferrous Sulfate: 25-kilogram bags, one-ton pallets
What Is Ferrous Sulfate?
Ferrous sulfate, known as a salt with the general formula FeSO4.xH2O, is a mineral compound. This mineral i commonly found in the form of hydrates and various water molecule configurations. Ferrous sulfate appears as a green-bluish salt and was known as “copperas.”
Ferrous sulfate is a stable chemical compound that exists as a solid at room temperature. It easily dissolves in water, and its solution is colorless. Ferrous sulfate is a reducing agent and can react with oxidizing agents.
Applications of Ferrous Sulfate:
Ferrous sulfate has a wide range of applications in various industries. Here, we will find some of the important uses of this chemical compound.
Application in Agriculture
Ferrous sulfate is one of the most important iron fertilizers used in agriculture. This compoud helps plants produce chlorophyll and iron absorption. It also helps improve soil pH. In agriculture, the use of ferrous sulfate fertilizer depends on the type of the product or orchard, and the exact amount required should be consulted with agricultural experts. Additionally, foliar spraying of ferrous sulfate is one of the fertilization methods used in specific conditions as a supplementary approach for agricultural products. For example, after the flowering of crops, the use of ferric sulfate is recommended.
Industrial Applications
Ferrous sulfate finds utility as a valuable mineral in various industries. Among its applications are industrial wastewater treatment, phosphate removal in wastewater treatment, and its use in dyeing and refining industries.
Medical Applications
Ferrous sulfate used as a significant source of iron in multivitamin and mineral supplements used in the field of medicine. Furthermore, it is used to treat iron deficiency (anemia).
Wastewater treatment Applications
Iron sulfate is utilized in various industries as a primary source of iron and a key substance in water treatment processes, for removing magnesium and calcium ions from water. On the other hand, it’s considered a coagulant in wastewater treatment. This mineral salt is also used to adjust the acidity of the alkaline environment.
Coloration and Printing
ferrous sulfate can be used as a pigment in the dyeing and printing industries.
Textile and Leather Industries
In some textile and leather production processes, ferrous sulfate used for fixing colors and darkening leather and hides.
Electronics and Computer Industry
In some electronic processes, ferrous sulfate serves as coating and resistance material. This chemical added to the cooling water that flows through the turbine condenser tubes to create a protective and corrosion-resistant layer.
Cement Industry
It utilized as a reducing agent to convert the chromium into a less harmful compound, chromium (III) in cement.
Production Method of Iron Sulfate
Iron(ll) sulfate is a chemical compound with diffrent applications in various industries. This substance is naturally found in some minerals like pyrite. Additionally, ferrous sulfate can be produced using various methods.
Pyrite Oxidation Method
One of the methods for producing ferrous sulfate is pyrite oxidation. Pyrite is a mineral containing iron and sulfur. In this process, pyrite exposed to air and oxidized. The iron present in pyrite is converted into iron sulfate.
Reaction Formula:
4FeS2 + 11O2 → 2Fe2O3 + 8SO2
Hydration Method
In this method, iron, along with water and sodium carbonate gradually added to an alkaline media, typically sodium hydroxide. This process slowly leads to the formation of iron sulfate. This method is commonly used in large mining and industrial facilities.
Reaction Formula:
Fe2O3 + 3H2O → 2Fe(OH)3
Dissolution Method
This method is one of the most common ways to produce ferrous sulfate. In this method, iron (Fe), in the form of powder or ground added to a mixture of sulfuric acid (H2SO4) and water.
Reaction Formula:
Fe + H2SO4 -> FeSO4 + H2
Smelting Process
In this method, iron, in the form of slag (concentrates), combined with sulfuric acid. The chemical reaction here involves the melting of the slag and the separation of iron sulfate from it.
Reaction Formula:
FeO + H2SO4 -> FeSO4 + H2O
Selective Precipitation
iron sulfate can be produced by selectively precipitating it from solutions by removing metals that are less reactive than iron.
CuSO4 + Fe ⟶ FeSO4 + Cu
Iron sulfate can be produced by diffrent methods. The choice of the appropriate production method depends on various factors such as production quantity, product quality, and production cost.







دیدگاهها
هیچ دیدگاهی برای این محصول نوشته نشده است.